Our group has been active in developing the organic chemistry, biochemistry, and applications of porphyrins and related macrocycles for more than three decades. Our synthetic work is exemplified by the discovery of facile SNAr reactions for porphyrins, the development of organometallic functionalization reactions, studies on N-substituted porphyrins, total synthesis of chlorins and synthetic methods for multi-porphyrin arrays.
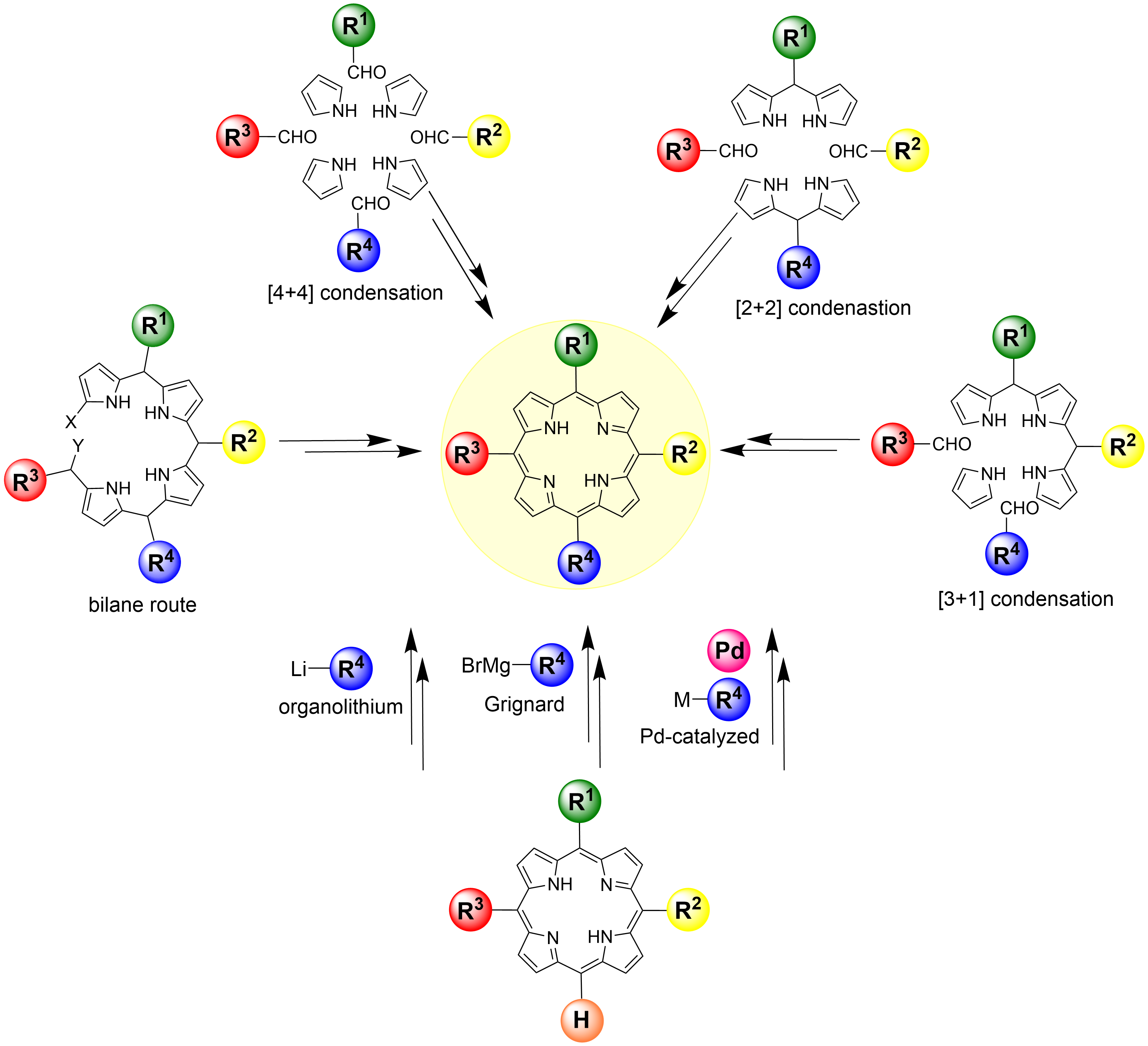
Retrosynthetic strategies to porphyrin synthesis

Synthesis from pyrrole starting materials
Currently, our group continues this legacy towards efficient and robust methods of accessing novel porphyrin modalities. Whilst symmetrical porphyrins such as 5,10,15,20-tetraphenylporphyrin are readily available through classic condensation routes, there is significant demand in medicinal chemistry and materials science for non-symmetrical porphyrins, which are more difficult to realize. Our group is continually developing highly versatile methods to synthesize unsymmetrical porphyrins with varying substitution patterns. By combining classical porphyrin synthesis with organolithium and modern metal-catalyzed organic reactions, porphyrinoid molecules with highly unusual substitution patterns can be obtained.
New synthetic strategies are being applied for the functionalization of naturally occurring porphyrins and the synthesis of bio-inspired compounds, as well as for the synthesis of highly distorted nonplanar porphyrins. These new synthetic methodologies developed in the group grant access to molecules with novel functionalities and exciting properties; for example, our recent study with freebase non-planar porphyrins acting as organocatalysts.

